-
Welcome to Smashboards, the world's largest Super Smash Brothers community! Over 250,000 Smash Bros. fans from around the world have come to discuss these great games in over 19 million posts!
You are currently viewing our boards as a visitor. Click here to sign up right now and start on your path in the Smash community!
It appears that you are using ad block :'(
Hey, we get it. However this website is run by and for the community... and it needs ads in order to keep running.
Please disable your adblock on Smashboards, or go premium to hide all advertisements and this notice.
Alternatively, this ad may have just failed to load. Woops!
Please disable your adblock on Smashboards, or go premium to hide all advertisements and this notice.
Alternatively, this ad may have just failed to load. Woops!
omg. Chem help Pleeeese :(
- Thread starter Alias
- Start date
Lixivium
Smash Champion
- Joined
- Mar 26, 2006
- Messages
- 2,689
We live in the wonderful age of the internets. All you had to do was type "5 isomers of hexane" into Google and go to the second link:
http://jchemed.chem.wisc.edu/JCESoft/CCA/CCA5/MAIN/1ORGANIC/ORG03/TRAM03/D/NOMOVIE/MISC.HTM
http://jchemed.chem.wisc.edu/JCESoft/CCA/CCA5/MAIN/1ORGANIC/ORG03/TRAM03/D/NOMOVIE/MISC.HTM
Wait.... what?i'll remember you <3

brawlpro
Smash Master
Listen to lixivium!
Zink
Smash Champion
Um, I don't think those are CH3 or CH2 groups. It's just Cs with H branches. Hexane means 6 carbons total, and 14 hydrogens.
MoogleDude
Smash Cadet
- Joined
- Jan 2, 2007
- Messages
- 61
i need help on my chem hw too plz!!!!
i know the steps to balancing redox reactions, (like split em in half) but i dont know how to like split these ones....
Cl2 + H2O -> OCl- + Cl-
H2O2 + NO2- -> NO3-
yah if ne1 could help me out... im desperate!
i know the steps to balancing redox reactions, (like split em in half) but i dont know how to like split these ones....
Cl2 + H2O -> OCl- + Cl-
H2O2 + NO2- -> NO3-
yah if ne1 could help me out... im desperate!
Zink
Smash Champion
The top one is messed up, there's no H produced...i need help on my chem hw too plz!!!!
i know the steps to balancing redox reactions, (like split em in half) but i dont know how to like split these ones....
Cl2 + H2O -> OCl- + Cl-
H2O2 + NO2- -> NO3-
yah if ne1 could help me out... im desperate!
Same with the second one actually...
They're the exact same thing, just written differently.Um, I don't think those are CH3 or CH2 groups. It's just Cs with H branches. Hexane means 6 carbons total, and 14 hydrogens.
It's not messed up, the protons simply aren't written out.The top one is messed up, there's no H produced...
Same with the second one actually...
First one's easy; Cl2 is being both oxidized and reduced, so the first reaction you want to look at is:Cl2 + H2O -> OCl- + Cl-
H2O2 + NO2- -> NO3-
Cl2 -> OCl-
When you balance it and all, you get Cl2 + H2O -> 2OCl- + 2e- + 4H+
Next we look at:
Cl2 -> Cl-
After balancing and such, we find that it is:
Cl2 + 2e- -> 2Cl-
Adding the two together, we get for our final equation:
2Cl2+ 2H2O -> 2OCl- + 2Cl- + 4H+
Second one's a little trickier, and it's been a while since I've done a lot of hydrogen peroxide redox to be honest so I can't remember how to completely balance it.
The nitrite part is easy though:
NO2- -> NO3-
When balanced yields: H2O + NO2- -> NO3- + 2e- + 2H+
I cannot seem to get the peroxide to balance properly unfortunately. Are you sure it's H2O2 and not just H2O?
Keep the chem questions coming though!
Yup, these are right. The way you would write the condensed formula for each is as follows (starting with the one on the upper left, then going down, then looking at the ones on the right and going down):Here I did it for you:
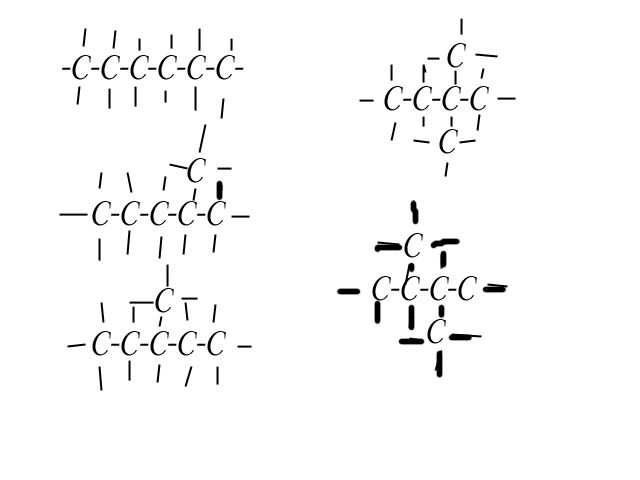
I'm not sure I did everything ok because it's been a while since my last science class.

I've also included the systematic name for each one, but those probably don't mean much to you at this point, heh.
MoogleDude
Smash Cadet
- Joined
- Jan 2, 2007
- Messages
- 61
wellz we went over it today and the teacher wuz like... since its an aqueous solution, theres like water molecules everywhere so its likeThey're the exact same thing, just written differently.
It's not messed up, the protons simply aren't written out.
First one's easy; Cl2 is being both oxidized and reduced, so the first reaction you want to look at is:
Cl2 -> OCl-
When you balance it and all, you get Cl2 + H2O -> 2OCl- + 2e- + 4H+
Next we look at:
Cl2 -> Cl-
After balancing and such, we find that it is:
Cl2 + 2e- -> 2Cl-
Adding the two together, we get for our final equation:
2Cl2+ 2H2O -> 2OCl- + 2Cl- + 4H+
Second one's a little trickier, and it's been a while since I've done a lot of hydrogen peroxide redox to be honest so I can't remember how to completely balance it.
The nitrite part is easy though:
NO2- -> NO3-
When balanced yields: H2O + NO2- -> NO3- + 2e- + 2H+
I cannot seem to get the peroxide to balance properly unfortunately. Are you sure it's H2O2 and not just H2O?
Keep the chem questions coming though!
H2O2 -> H2O
then... yah the ez stuff
i wuz only confused on the first one cus of that H2O (didn't if you hadda make a half reaction for it or not)
mark.
Smash Journeyman
i super aced regents chem :D
but since somebody answered it lol
but since somebody answered it lol

